Aromatic Ketons :-
Those organic compound in which carbonyl group attached to aryl group or one alkyl and one aryl group are called aromatic ketones.
Methods of Preparation of Acetophenone :-
- Friedal-Crafts Methods - When benzene is heated with acetylchloride or acetic anhydride in presence of anhydrous AlCl3 , Acetophenone is obtained .
- By Catalytic Oxidation - When Ethyl benzene is oxidised by air or oxygen in presence of Magnes acetate at 120°C , Acetophenone is formed.
Physical Properties
- It is colourless liquid and has B. P. 240°C.
- Ist is less soluble in H2O but in alcohol and ether it is soluble.
Chemical Properties
In Acetophenone, One methyl and one phenyl group atteched with carbonyl group. Hence it gives all aliphatic Ketones reaction.
Effect of -COCH3 is m-directing. Hence it gives all electrophillic substitution reaction like benzene. Nucleophillic addition reaction of -COCH3 are as follows-
- Addition of HCN-
Acetophidron give additional reaction with hydrocynic acid and form acetophenon cynohydrin.














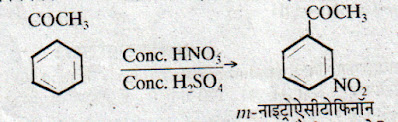


YES , लेकिन अभी समय लगेगा। हिन्दी के लिए काम किया जा रहा हैं।
जवाब देंहटाएं